
The U.S.
Department of Energy requests that no alterations be made without permission in
any reproduction of this report.
Ozone Treatment for Cooling Towers
New Energy and Water Saving
Technology to Reduce Cooling Tower Operating Costs
Abstract
The use of ozone as a
maintenance treatment for cooling towers has good potential for operation and
maintenance savings in the Federal sector. A small amount of ozone acts as a
powerful biocide that decreases or nearly eliminates the need to remove
quantities of water from the cooling tower in order to decrease the
concentration of organic and mineral solids in the system. Ozone treatment can
also reduce the need for chemical additives added to the cooling tower water.
In a properly installed and
operating system, bacterial counts are reduced, with a subsequent minimization
of the buildup of biofilm on heat exchanger surfaces. The resulting reduction
in energy use, increased cooling tower operating efficiency, and reduced
maintenance effort provide cost savings as well as environmental benefit and
regulatory compliance with respect to discharge of wastewater from blowdown.
Cooling towers associated
with chillers for air-conditioning are good candidates for ozone application.
Ozone may be a corrosion stimulant rather than an inhibitor, and this can be a
factor in some circumstances. Nevertheless, it is easier to combat corrosion in
a clean system than in one that is biologically and mineralogically fouled.
This Federal Technology
Alert (FTA) provides detailed information and procedures that a Federal
energy manager needs to evaluate most cooling tower ozone treatment
applications. The New Technology Demonstration Program (NDTP) technology
selection process and general benefits to the Federal sector are outlined.
Ozone treatment, energy savings, and other benefits are explained. Guidelines
are provided for appropriate application and installation. Two actual case
studies are presented to give the reader a sense of costs and energy savings.
Current manufacturers, technology users, and references for further reading are
included for prospective users who have specific or highly technical questions
not fully addressed in this FTA.
Ozone
is a molecule consisting of three oxygen atoms and is commonly denoted O3.
Under ambient conditions, ozone is very unstable and as a result has a
relatively short half-life of usually less than 10 minutes. Ozone is a powerful
biocide and virus deactivant and will oxidize many organic and inorganic substances.
These properties have made ozone an effective chemical for water treatment for
nearly a century. During the last 20 years, technological improvements have
made smaller-scale, stand-alone commercial ozone generators both economically
feasible and reliable. Using ozone to treat cooling tower water is a relatively
new practice; however, its market share is growing as a result of water and
energy savings and environmental benefits relative to traditional chemical
treatment processes. A typical system for ozone treatment of cooling towers is
shown in Figure 1. Ozone treatment of cooling tower water is not feasible in
all situations and hence traditional chemical treatment of cooling tower water
is the *only alternative.
*Electorcoagulation is the only serious alternative to
ozone treatment for removal of
minerals and water purification . See Ecoloquip Inc., catalogue.
Fig. 1. Typical Cooling
Tower Ozone Generation System
A
cooling tower functions to cool a circulating stream of water (see Figure 2).
The tower acts as a heat exchanger by driving ambient air through falling
water, causing some of the warmed water to evaporate (evaporation gives off
heat--providing cooling), and then circulating cooler water back through
whatever equipment needs cooling (such as a chiller condenser). Typically,
chemicals such as chlorine and chelating agents are added to cooling tower water
to control biological growth (called "biofilm") and inhibit mineral
build-up (called "scale"). The control of biofilm and scale is
essential in maintaining cooling tower heat transfer efficiency. As the water
volume in the tower is reduced through evaporation and drift, the concentration
of these chemicals and their byproducts increases. Cooling towers also pick up
contaminants from the ambient air. To maintain chemical and contaminant
concentrations at a prudent level, water is periodically removed from the
system through a process called "blowdown"or "bleed off".
The blowdown water and the water lost through evaporation and drift are
replaced with fresh "make-up" water (which will also contain minerals
and other impurities).

Fig. 2. Typical Cooling
Tower Operation
Blowdown
water must subsequently be discharged to a local wastewater treatment facility
or discharged onsite to the environment. The blowdown water typically contains
little organic material, and the local wastewater treatment facility will
charge extra sewage fees for accepting the water. These costs can be quite
significant in the overall costs of operating a cooling tower. Discharge of the
blowdown water to the environment onsite is coming under increasing regulation
due to stricter regulation of the contaminants typically found in blowdown
water. Ozone will dissipate quickly and not be found in the blowdown water.
This reduces the overall chemical load found in the discharged water, making it
easier to comply with regulations.
Most
cooling tower ozone treatment systems include the following components: an air
dryer, air compressor, water and oil coalescing filters, particle filter, ozone
injectors, an ozone generator, and a monitoring/control system. Ambient air is
compressed, dried, and then ionized in the generator to produce ozone. Ozone is
typically applied to cooling water through a side stream of the circulating
tower water as is illustrated in Figure 3.

Fig. 3. Process for Ozone
Treatment of Cooling Tower Water
Field
tests have demonstrated that the use of ozone in place of chemical treatment
can reduce the need for blowdown, and, in some cases where make-up water and
ambient air are relatively clean, can eliminate it. As a result, cost savings
accrue from decreased chemical and water use requirements and from a reduction
of wastewater volume. There are also environmental benefits as fewer chlorine
or chlorinated compounds and other chemicals are discharged.
There
is also a belief within the industry (and some evidence) that under certain
conditions ozone acts as a descaling agent. The premise is that ozone oxidizes
the biofilm that serves as a binding agent adhering scale to heat exchange
surfaces. When scale buildup on condenser tubes is reduced, higher heat transfer
rates are achieved. Increasing the condenser heat transfer rate will reduce the
chiller head pressure, which then allows the chiller to operate more
efficiently and consume less energy.
There
is a growing number of manufacturers and distributors of ozone equipment in the
United States, and the use of this technology is encouraged by several major
electric utilities and by electric utility and cooling tower associations. Each
new application of ozone for cooling tower water treatment increases understanding
of its overall effectiveness and its applicability under differing physical
conditions. The technology has had both success and failure.
More
information on the criteria for applicability and the potential for the use of
this technology in the Federal sector is provided below.
Application Domain
It
is estimated that ozone treatment is applied on anywhere from 300 to 1,000
cooling towers in the United States. Most of these towers dissipate heat
generated by commercial heating, ventilating, and air-conditioning (HVAC)
systems and light industrial processes. The total number of cooling towers
requiring chemical treatment in the United States is estimated at between
500,000 and 600,000.
Biological
growth, scaling, and corrosion are the main maintenance concerns with these
cooling towers. Typical treatment involves the application of chemicals such as
chlorine, sulfuric acid, phosphorous, and zinc compounds. Care must be taken in
the storage, use, or discharge of these chemicals. Care must be taken to ensure
that the proper mixes and proportions of chemicals are used, and to determine
the corresponding blowdown rates. Excessive application can increase the
possibility of corrosion and other undesirable impacts. As traditional chemical
water treatments are being restricted because of environmental concerns, ozone
is gaining acceptance as a viable biocide alternative.
Cooling
tower water is continuously exposed to airborne organic materials, and the
buildup of bacteria, algae, fungi, and viruses presents hazards to the tower
system and to the health of humans encountering the water. For example,
Legionnaire's Disease is caused by the bacterium Legionella pneumophila
that frequently thrives in cooling tower environments. High levels of bacteria
can also lead to an increased risk of microbial influenced corrosion. Certain
sulfate-reducing and iron-metabolizing bacteria can destroy iron piping in as
little as 9 months. Moreover, a biofilm coating on heat exchanger surfaces
reduces heat transfer efficiency. Ozone kills bacteria by rupturing their cell
walls, a process to which microorganisms cannot develop immunity. Residual
ozone concentrations greater than or equal to 0.4 mg/L have been shown to
result in a 100% kill in 2 to 3 minutes for Pseudomonas fluorescens (a
biofilm producer) in a biofilm, while residual concentrations of as little as
0.1 mg/L will remove 70-80% of the biofilm in a 3-hour exposure. Studies have
also shown that ozone concentrations less than 0.1 mg/L will reduce the
populations of Legionella pneumophila in cooling tower waters by 80%.
Another
phenomenon requiring treatment in cooling towers is mineral buildup. Minerals
such as calcium and magnesium, which are common dissolved solids in water, are
deposited by two different mechanisms, thermal and biological. As the water in
a tower evaporates, dissolved solids concentrate in the recirculating water.
Biofilms also start to form on the walls and other components of the tower. In
essence, the biofilm acts as an adherent for mineral micro-crystals. Over time,
deposition of organic and inorganic matter increases scale thickness. Ozone can
loosen and remove the scale if the biofilm is present, but if the biofilm is
not present the ozone may be ineffective in removing the scale. Biofilm may not
be the dominant fraction of scale where the temperature of the heat exchanger
is in excess of 135 F. Scale-forming minerals are less soluble at these higher
temperatures and can deposit from solution directly onto pipe walls.
One
operating concern of a cooling tower is the gradual corrosion of various parts
of the tower. Much of the corrosion in cooling towers is associated with
bacteria that create conditions favoring microbiologically induced corrosion.
When adequate quantities of ozone are injected, control of the microbial
population is accomplished. On the other hand, due to its high chemical
oxidation potential, ozone can be quite corrosive. However, because a very
small amount of ozone performs effectively as a biocide, and because of its
very short half-life, the corrosive effects are minimized.
There
is also an observed phenomenon of ozone-treated cooling tower water, wherein
the pH of the system rises above 8.5 and corrosion protection of the cooling
tower components takes place. This phenomenon may also be dependent upon
make-up water characteristics such as alkalinity and hardness, so it does not
release the operator of the cooling tower from the obligation of making regular
corrosion measurements.
Energy and Water Saving
Mechanisms
Scale
and biological deposits reduce the ability of refrigerant condensers and
industrial-process heat-exchangers to transfer heat. By removing and inhibiting
biological deposits and scale more effectively than chemical treatment, ozone
cooling tower water treatment can improve chiller system performance.
Manufacturers claim an average efficiency gain of 10%; case studies range from
no improvement in efficiency to a 20% improvement in chiller performance.
Energy savings should be estimated for each individual application and based on
the actual operating condition of the condenser or heat exchanger and the type
of scale present. Further, any projected electrical savings must be weighed
against energy consumed by ozone generators and auxiliaries, typically 9 kWh to
14 kWh per pound (0.45 kg) of ozone generated.
Water
is lost from a cooling tower in three ways: drift, evaporation, and blowdown.
Drift occurs when the water droplets become entrained in the discharge
airstream and can be controlled through cooling tower design. Evaporation is
from air passing through the cooling water and absorbing heat and mass.
Blowdown is intentional bleed-off (replaced by make-up water) to reduce the
concentration of contaminants.
The
capacity of a cooling tower is typically measured in tons, the rate at which
the tower rejects heat. One ton of cooling is equal to rejecting 12,000 Btu
(British thermal units) per hour (3.5 kW). This heat is released through
evaporation. The rate of evaporative water loss is about 12 gallons (45.4 L)
per minute for every 500 tons (1,750 kW) of cooling tower tonnage. Ozone will
not increase or decrease the rate of evaporation. However, compared to chemical
treatment at the allowable dosages, ozone treatment contributes far less to the
tower's dissolved solids loading in the circulation water and is therefore more
amenable to operation at higher cycles of concentration.
"Cycles
of concentration," "number of cycles," or "concentration
ratio" are some of the terms used to describe the relationship between the
quantity and quality of make-up water and the volume and constituents of the
bleed-off. This concentration ratio can be thought of as an indicator of the
number of times water is used in the cooling tower before it is discharged,
based on a mass balance between dissolved solids entering the system in make-up
water and dissolved solids leaving the system in blowdown. The higher the
cycles of concentration, the lower the blowdown.
Blowdown
water from a cooling tower can be sent to a municipal drain, or it may require
onsite pretreatment prior to disposal to a drain. In some cases, blowdown may
be stored onsite and then retrieved by a disposal service. The savings are a
direct function of the costs associated with these three disposal processes and
the blowdown volume reduction achieved by the ozone system.
If
water and sewer services are purchased from a municipal or public utility,
reducing blowdown and make-up water requirements will trigger a series of
resource and cost savings for those municipal utilities. If the site operates
its own water treatment and wastewater treatment facilities, reducing blowdown
and make-up water requirements will allow the facility to realize these
benefits directly as follows:
- reduced
pumping power to extract water from source wells or reservoir and pump to
water treatment facility
- reduced
chemical, filtration, and maintenance costs associated with treating and
purifying at the water treatment facility
- reduced
pumping power for distributing the water from the water treatment facility
to the end-user
- reduced
pumping power and associated costs to transport wastewater (blowdown) to
the sewage treatment plant
- reduced
chemical and maintenance costs, and reduced pumping power associated with
sewage treatment at the plant
- reduced
costs associated with permits allowing the discharge of treated sewer
water to a river or stream.
Other Benefits
Besides
its potential to reduce water and energy requirements, ozone treatment can
reduce or eliminate chemical use, eliminate infectious bacteria, and improve
regulatory compliance. Environmental and health benefits occur as potentially
harmful molecules are broken down into less toxic byproducts. Properly
controlled ozone applications decrease the levels of both bacterial and mineral
substances in the waters discharged through blowdown or bleed-off.
Chemical
treatment costs vary according to the size and chemical requirements of the
tower. These costs can be reduced by using ozone as the treatment technology.
Case studies indicate that chemical cost savings are a large contributor to the
cost-effectiveness of an ozone system.
One
manufacturer claims that in normal operation, chiller tubes are usually brushed
out once a year, and the tower sump is shoveled once or twice per year. When
performing a cost savings evaluation for a potential customer, the manufacturer
takes credit for eliminating this maintenance requirement. Although it may not
be necessary to brush out the tubes more than once a year, it may still be
necessary to shovel the sump for a number of possible reasons. Therefore, it is
generally recommended not to accept maintenance and labor savings estimates for
a facility without consulting the facility's maintenance personnel. In
addition, it is more likely that maintenance savings will come from the
reduction in chemical treatment system labor. This savings should be weighed
against maintenance requirements of the ozone system, which are reported to be
minor.
Finally,
with a reduction in biological growth, scale, corrosion, and chemical use, the
issue of liability decreases as well. From a human resources perspective,
reduced risk to personnel health enhances the working environment and makes a
positive public statement.
Variations
Ozone
generation is accomplished by passing a high-voltage alternating current
(6-20kV) across a dielectric discharge gap through which air is injected (see
Figure 4). As air is exposed to the electricity, oxygen molecules disassociate
and form single oxygen atoms, some of which combine with other oxygen molecules
to form ozone. Different manufacturers have their own variations of components
for ozone generators. Two different dielectric configurations exist--flat
plates and concentric tubes. Most generators are installed with the tube
configuration. Cylindrical configurations offer the easiest maintenance.
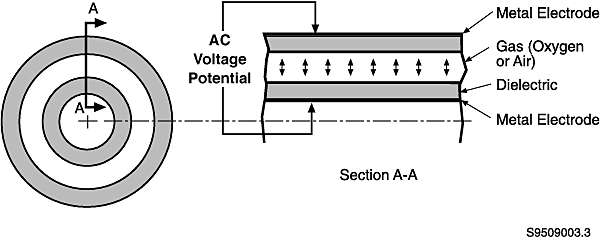
Fig. 4. Dielectric Process
for Ozone Generation
Mass
transfer of the ozone gas stream to the cooling tower water is usually
accomplished through a venturi in a recirculation line connected to the sump of
the cooling tower where the temperature of the water is the lowest. Since the
solubility of ozone is very temperature-dependent, the point of lowest
temperature provides for the maximum amount of ozone to be introduced in
solution to the tower. Mass transfer equipment can take other forms: column
bubble diffusers, positive pressure injection (U-tube), turbine mixer tank, and
packed tower. The counter-current column-bubble contactor is the most efficient
and cost-effective but is not always useful in a cooling tower setting because
of space constraints. Hence, setups like a venturi followed by an in-line
static mixer, or an eductor followed by an in-line static mixer, are common in
the installation of an ozone system.
Some
ozone treatment equipment vendors propose that the most effective use of ozone
is through controlled low doses proportional to the thermal and organic loads
of the water. Several factors can influence load, or the oxidation reduction
potential (ORP) of the water, including temperature, air quality in the
vicinity of the tower, and cooling demands. To provide a proportional quantity
of ozone, the ORP must be measured frequently and the ozone generation system
must be capable of instant response to changes in ORP. The ORP is a useful
criterion because other biocides can accumulate in the tower when blowdown is
reduced. These biocides include chlorine from the make-up water and bromate
species resulting from the ozone oxidation of trace bromine in the make-up
water.
Unfortunately,
the ORP probe is prone to fouling (usually by a fine layer of calcium
carbonate). Maintenance is simple--and it is essential. If the probe is not
cleaned, the ozone system is likely to stray from proportional control. The
benefit of proportional control and variable ozone generation capability is
that only the necessary quantity of ozone is generated; thus, energy
consumption costs are minimized, as is the possibility of corrosion from
excessive ozone.
Ozone
generators create heat and require a cooling system. Some manufacturers
indicate that water is the coolant of choice; however, others prescribe cabinet
air-conditioning units to keep constant temperatures and reduce air moisture
content. Regardless of which system is employed, reliable cooling is essential
to preserve the dielectric and to optimize ozone generation.
The
potential cost-effective savings achievable by this technology were estimated
as a part of the technology assessment process of the New Technology
Demonstration Program (NTDP).
Technology Screening Process
New
technologies were solicited for NTDP participation through advertisements in
the Commerce Business Daily and trade journals, and through direct
correspondence. Responses were obtained from manufacturers, utilities, trade
associations, research institutes, Federal sites, and other interested parties.
Based on these responses, the technologies were evaluated in terms of potential
Federal-sector energy savings and procurement, installation, and maintenance
costs. They were also categorized as either just coming to market
("unproven" technologies) or as technologies for which field data
already exist ("proven" technologies). Note this solicitation process
is ongoing and as additional suggestions are reviewed, they are evaluated and
become potential NTDP participants.
The
energy savings and market potentials of each candidate technology were evaluated
using a modified version of the Facility Energy Decision Screening (FEDS)
software tool, developed for the Federal Energy Management Program (FEMP),
Construction Engineering Research Laboratories (CERL), and the Naval Facilities
Engineering Service Center (NFESC) by Pacific Northwest National Laboratory
(PNNL) (Dirks and Wrench 1993).
During
the solicitation period in which ozone treatment of cooling tower water was
suggested, 21 of 54 new energy-saving technologies were assessed using the
modified FEDS. Thirty-three were eliminated in the qualitative pre-screening
process for various reasons: not ready for production, not truly energy-saving,
not applicable to a sufficient fraction of existing facilities, or not U.S.
technology. Eighteen of the remaining 21 technologies, including ozone
treatment of cooling tower water, were judged life-cycle cost-effective (at one
or more federal sites) in terms of installation cost, net present value, and
energy savings. In addition, significant environmental savings from use of many
of these technologies are likely through reductions of CO2, NOx
and SO2 emissions. Several of these technologies that have a
demonstrated field performance have been slated for further study through Federal
Technology Alerts.
Laboratory Perspective
Through
laboratory testing, field testing, and theoretical analysis, ozone treatment of
cooling tower water has shown to be technically valid and economically
attractive in many applications. The technology works by virtue of the ability
of ozone to act as a disinfectant and therefore as an alternative to
traditional chemical treatment. Performance of the technology, when properly
applied, has been demonstrated effective. However, like most traditional
chemical treatment programs, ozone is not a cure-all. Ozone is a potential
alternative to traditional chemical treatment methods. More information is
needed on the effectiveness, efficiency and potential other impacts of ozone.
The remaining barriers to implementation involve user acceptance and correct
application. This Technology Alert is intended to address these concerns
by reporting on the collective experience of ozone users and evaluators and by
providing application guidance for Federal-sector installations.
This
section addresses technical aspects of applying ozone treatment technology to
cooling towers. The most appropriate applications are discussed.
Application Screening
To
determine whether ozone is an effective alternative for treating the water in a
specific cooling tower, a technical feasibility screening study and economic
(life-cycle cost) analysis should be performed. In general, cooling towers
associated with chillers for commercial HVAC and light industrial process
cooling are good candidates. Manufacturers claim to have treated both wooden
and metal towers in sizes ranging from 60 to 10,000 tons (210 kW to 35,000 kW).
A list of manufacturers is provided later in this Technology Alert.
Ozone
is not a corrosion inhibitor; however, the higher concentration ratios
resulting from the reduced blowdown volumes raise the pH of the recirculating
water, which helps protect the system from corrosion. This same pH condition
will promote the precipitation of silicates and calcium carbonate if sufficient
pretreatment of make-up water is not provided. Lower pH will remove the scale
but will also increase the corrosion rate from the ozone. For this reason,
make-up water must be of sufficient quality to avoid these problems.
The
strong oxidation potential of ozone is what makes it most attractive for use as
a biocide in water systems. However, this same property also makes it difficult
to use ozone when there is a large chemical oxygen demand (COD) present (this
will consume available ozone) in the water or if local air conditions bring in
large quantities of organics to the tower. The latter condition is the reason
it is not possible to implement ozone water treatment in towers within chemical
plants or at oil refineries. In addition, ozone is corrosive to some materials
such as rubber fittings, gaskets, and certain kinds of metals and alloys. If
these materials are present in a cooling tower, they should be replaced before
ozone system installation if it is practical and economical to do so.
Once
ozone is in the liquid phase, it will last only a short period of time; thus,
maintaining an ozone residual for more than approximately 10 minutes can be
difficult. This limits the application of ozonation in large cooling towers. In
large towers with 100,000 or more gallons, multiple injection points may be
necessary.
Make-up
water that is high in mineral content or dissolved solids may not be conducive
to effective treatment; testing should take place before a system is installed
and on a periodic basis during operation. A side-stream filter may be required
on cooling towers operating with make-up water quality in excess of 150 ppm
calcium hardness. In cases where hardness is in excess of 500 ppm as CaCO3,
or sulfates >100 ppm, ozone can be eliminated as a viable cooling tower
water treatment. A "Cooling Tower Worksheet" is provided in Appendix
A and can be used to characterize the quality of make-up water.
The
U.S. Occupation Safety and Health Administration (OSHA) has established an
ozone exposure limit of 0.1 ppm in air over an 8-hour shift. This could be a
problem if the cooling towers are located on the ground level and are
excessively treated with ozone so that the tower is operating as an ozone gas
stripper (gives off ozone into the air).
Ozone
produces oxidation by-products. There are several secondary products that must
be accounted for in the set-up of cooling tower ozonation. Both iron and
manganese will be oxidized by the ozone to form insoluble particulates that
collect in basins, on screens, or in any scale that is forming. Excessive
amounts of either of these two chemicals in the make-up water will require
pretreatment. In addition, organic compounds that may either be in the make-up
water or introduced through the atmosphere will react with ozone to form
ketones, aldehydes, and amines. If bromide is present, ozone can convert
bromide to hypobromous acid and hypobromite ion. These two species are known
biocides and would be considered helpful in controlling biofilms but
potentially detrimental in the discharge of blowdown. Excessive ozone can
further oxidize the hypobromite ion to bromate, reducing the effectiveness of
these components as biocides.
What to Avoid
Ozone
treatment failures are usually related to an inadequate quantity of
applied/dissolved ozone which can be caused by excessive organic material in
the water or high operating temperature. Therefore, ozone treatment should be
avoided in the following situations:
- high organic
loading from air, water, or industrial processes that would require a high
COD (the ozone will oxidize the organics and insufficient residual may
remain for the water treatment)
- water
temperatures that exceed 110 F (43.3 C) (high temperatures decrease ozone
residence time and reduce overall effectiveness of the ozone treatment)
- make-up
water is hard (>500 mg/L as CaCO3) or dirty make-up water
(softening and/or prefiltering make-up water is sometimes recommended)
- long piping
systems which may require long residence time to get complete ozone
coverage (insufficient ozone residence time may result in incomplete
coverage)
Water
temperature is critical to the success or failure of a system. Above 110 F
(43.3 C) the solubility of ozone is effectively zero for all concentrations of
ozone in the feed gas. Even at 104 F (40 C) the solubility is very small (<3
mg/L). Although some operational data suggest that ozone may be used at
temperatures of up to 135 F (57.2 C), most sources agree that ozone works best
in bulk water temperatures under 104°F (40°C), preferably even below 100°F
(37.7°C). Many comfort cooling systems commonly operate at between 85°F and
95°F (29.4°C and 35.0°C). As temperatures rise, the ozone will dissipate too
fast and not dissolve into the water. This is one reason ozone is not
appropriate for cooling tower systems such as nuclear and fossil generating
plants and absorption refrigerant plants, where temperatures are generally
high.
Problems
can and do occur in the field. The following precautions are not always covered
in manufacturers' instructions but are recommended to be taken during
installation:
- Preparation
of the inlet air is very important for the efficient operation of an ozone
unit as well as for the longevity of the unit. The preparation of the gas
includes removal of dust (particle sizes >1ตm),
moisture (dewpoint <-76° F (-60°C)="99.98%" moisture
removed), and oil. This requires that the pretreatment system be checked
periodically by properly trained personnel and that the appropriate
monitoring equipment for the pretreatment process is installed.
- Make-up
water should be free from noticeable sediment, mud, and discoloration and
should not have extremely high levels of sulfates (<100 ppm) or
hardness (<500 ppm as CaCO3). These values may be determined
by having the water tested by a qualified lab.
- Material in
the ozone-treated system should be compatible with ozone. The ozone
distribution line from the generator to the gas/water contactor carries
the highest concentration (1 to 4% by weight of ozone); therefore, the
line material should be constructed of stainless steel or PVC.
- For
efficient operation, the ozone generator should be located in an
air-conditioned area. Excessive heat (greater than 90 F) could damage the
system or reduce generation capacity.
- The actual
capacity of the ozone generator should be certified by the manufacturer
and checked yearly by the ozone vendor or a qualified maintenance
contractor.
- Corrosion
coupons for copper and steel should be placed in the system and checked at
least every 6 months.
Normally
the cooling tower manufacturer or vendor furnishes operating and maintenance
manuals and training. Manufacturers' instructions should continue to be
followed after the system is installed.
Quantitative Measurements
Ozone
concentration in the water can be measured. The measurement of ozone concentration
has been a source of some debate in the past. Two measurement methods are in
use today that are fairly well accepted. These are Absorption of UV light as
determined by the Beer-Lambert Absorption Law (OREC) and the Indigo method 8311
of HACH Company. The UV absorption method is useful for on-line monitoring of
the ozone concentrations in systems for cooling tower water treatment.
A
useful indicator of scaling is proposed by Pryor and Buffum, called Practical
Ozone Scaling Index (POSI). This index is a correlation for traditional scaling
indices for use in ozone treated systems. Tierney, Feeney, and Mott propose
examining the solubility based on activity coefficients as a function of ionic
strength using the DeBye-Huckel equation. This latter approach is a direct
assessment of scaling under super saturated conditions.
Equipment Integration
The
ozone systems for cooling tower application on the market today are typically
modular and fully self-contained systems with an independent circulation system
for sidestream installation. Ozonators operate from line voltage of 120 volts
single-phase, 230 volts single- and three-phase, and 440 volts single- and
three-phase, at 60 Hz. The higher the output, the more desirable it is to
operate from a higher voltage and multi-phase source. Electric service breakers
are system-mounted for single-point electrical connection. Units can arrive
completely wired and piped, with all components mounted on structural steel
skids (see Figure 5).

Fig. 5. Ozone System,
Showing Piping and Skids
The
necessary piping (usually PVC) and circulation pumps must be provided to
connect the system to the cooling tower water sump. Sometimes, filters must be
installed to capture mineral deposits that will occur from the ozone treatment.
Installation can typically be completed in one day provided the appropriate
electrical service is in place.
Monitoring
and control packages can include integral alarms. Also, interlocking features
are available so that remote fans, blowers, pumps, solenoid valves, etc. will
be activated upon start up of the ozonator and vice versa.
Different
ozone systems have different dimensions or "footprints." A system
designed to treat a 1,000-ton (3,500-kW) tower may have width-height-depth
system dimensions of 37 x 32 x 55 inches (0.94 x 0.81 x 1.4 meters) to 90 x 60
x 30 inches (2.3 x 1.5 x 0.76 meters). To maximize the use of ozone during its
short half-life, the ozone-containing water should be returned to the sump of
the cooling tower as close as possible to the suction side of the circulation
pumps, to ensure that the maximum amount of oxidant is circulated through the piping
and heat exchangers and that some ozone remains to be returned to the top of
the cooling tower.
Maintenance
As
with any technology, it is important to perform routine maintenance in order to
preserve overall efficiency and effectiveness, as well as to extend equipment
life. Preventive maintenance recommendations are listed in Table 1.
Table 1. Recommended
Preventive Maintenance
|
Frequency
|
Description
|
|
Three months |
Check/change filters |
|
Six months |
Change brushes on powerstat control |
|
Annually |
Check dielectrics |
|
Other |
Check air dryer pre- and post-filter as
specified by air dryer manufacturer |
Warranties
Ozone
technology appears to be a reliable method for cooling tower water treatment.
As with any water treatment process, there are reported successes and failures.
As with most equipment, warranties vary between manufacturers. Although a full
comparison of warranty information cannot be provided in this Technology
Alert, one manufacturer warrants the electrodes in the ozone generator for
three years.
The
reader should inquire into the ozone equipment warranty directly from the ozone
equipment manufacturer or sales representative. In addition, the reader should
inquire into the impact on the chiller and cooling tower equipment warranties
directly from the providers of the chillers and cooling towers. Some ozone
technology providers disclaim any warranty with regard to the use of the ozone
equipment. The actual terms of the warranty are usually set forth in the
specification submittal or documents of sale. The reader is encouraged to
investigate the equipment warranties.
Costs
Costs
for a typical ozone system capable of treating a 1,000-ton (3,500-kW) cooling
tower are estimated to range from $25,000 to $70,000, depending upon
manufacturer and actual system size. $36/ton of cooling may be used to provide
a rough cost estimate for an ozone system. The ozone systems are sized
according to need and range from 10 gr/hour to 3,700 gr/hour with corresponding
prices ranging from $10,000 to $300,000. The wide range in cost is a result of
the fact that the size, and subsequently the cost, of the system depends
heavily upon the operating temperature and operating environment of the tower.
Utility Incentives and Support
Although
no utilities currently offer rebates for ozonation, a number have sponsored
seminars and disseminated information. Some have sponsored field tests and
comprehensive studies. The reader is urged to contact your local utility to see
if any energy savings rebates are available.
Texas
Utilities (TU) has worked with one company since spring of 1994 and has
completed four ozone installations for TU customers. Southern California Edison
has studied installations and offers information to its customers. Pacific Gas
& Electric evaluated a test installation over a two-year period and
concluded that ozone was "superior to the current, conventional,
multi-chemical treatment program." Georgia Power, Alabama Power, and the
TVA all sponsored onsite seminars on cooling tower ozonation for their
customers in 1994.
A
large number of case studies have been reported by manufacturers and others.
Observations of field performance, obtained from Federal- and private-sector
analysts and users, are summarized below.
Pacific
Gas and Electric reported effective use of ozone as a biocide following a
2-year study of treatment of mechanical draft counterflow water cooling towers
at a large gas production utility site.
An
Electric Power Research Institute (EPRI) case study focuses on the Digital
Equipment Corporation offices in Littleton, Massachusetts, a 500,000
square-foot complex. The ozonation system was commissioned in 1989. Digital
engineers found ozonation to be economically and environmentally superior to
previous chemical treatments. In addition to the biocidal effect, ozonation
reduced blowdown and eliminated the need for employees to handle chemicals.
Tests over 2.5 years showed no scale formation; corrosion rates were within
industry standards and equipment manufacturer recommendations. Operating costs
were reduced by almost $90,000 per year, and the payback period for capital
investment was only about 2 years.
In
1984-85, NASA performed an experiment in which a 600-ton cooling tower was
retrofitted with an "Ozone-Air HF-90" solid-state ozone generator,
which used 60% less electricity to make a pound of ozone than a conventional
transformer/glass-electrode generator (6.1 vs. 15.3 kWh/lb ozone). The
generator cost a total of $16,057 for a 2-cfm air compressor, air dryer, and
ozone generator. Its use decreased the cooling tower's bacterial count by four
orders-of-magnitude and turbidity by eightfold. Scale accumulations on the
tower loosened and fell off. The effect on chiller energy consumption was not
measured, but the condensers were found to be clean and looking as though they
were newly retubed. Negative impacts included ozone attack on galvanized steel,
copper, and nylon fittings; these were eventually replaced with PVC and
stainless steel.
This
case study examines a system of four ceramic-filled concrete cooling towers
with a capacity of 2,500 tons (8,750 kW) each. The towers reject heat from the
air-conditioning system that provides temperature and humidity control for
Space Shuttle processing in the Vehicle Assembly Building (VAB) at NASA's
Kennedy Space Center (KSC), Florida.
Facility Description
The
cooling towers that provide service to the VAB are located in the Utility Annex
(central plant) at KSC. The make-up water is purchased from a Privately Owned
Treatment Works (POTW) at a cost of $1.18/1,000 gallons ($0.31/1,000 liters)
and blowdown was discharged to local surface waters. Chemical treatment for the
cooling tower was $10.18/ton per year ($2.91/kW) and consisted of two phase
scale and corrosion inhibitors and alternating biocide application. In 1990,
the Florida Administrative Code (FAC) 17-302, Surface Water Quality Standards,
introduced stricter environmental regulations that made the blowdown water
unable to meet regulatory criteria for discharge to the local surface waters.
Hence, ozone treatment was installed in February 1994 in an attempt to reduce
the amount of blowdown being discharged.
Existing Technology Description
The
four cooling towers have a total capacity of 10,000 tons (35,000 kW) and
contain a total of 204,000 gallons (772,000 liters) of cooling water. The
towers had an average make-up water volumetric rate of 146,000 gal/day (553,000
liters/day). Blowdown averaged 67,200 gal/day (254,500 liters/day) with the
rest being a combination of drift and evaporation. The towers reportedly were
operated with a concentration ratio in the range of 4 to 7. Cooling water is
circulated at 7,500 gal/min (28,400 liters/minute) through each tower. The
tower water temperature drops from 110 F (43.3 C) to 90 F (32.2 C).
Ozone Equipment Selection
Ozone
vendors have well-developed specifications for the implementation of
ozone-producing equipment. These criteria consider all aspects of the system.
Many factors must go into the decision to use ozone as a cooling tower water
treatment. Among these factors are the operating environment, operating
temperature, material resistance to ozone, and condition of the make-up water.
However, it is important to have an estimate of the size and cost of an ozone
system before contacting a vendor.
The
size, cost, and operating conditions of the existing system should be obtained
so that a comparison can be made with using ozone. If this information is not
available, the inputs needed may be estimated in the Cooling Tower Worksheet.
It is necessary to know the nominal rating of the cooling tower(s) under
examination. Cooling tower capacity is usually expressed in terms of tons. Once
the tower capacity is obtained, the system can be sized using the equations
identified in the Cooling Tower Worksheet, as shown in Figure 6.

Savings Potential
A
preliminary analysis will provide estimates that will be useful in making a
decision to implement ozone as a treatment for cooling tower water. The
estimation of the size and cost of an ozone system can be done at several
levels of detail. The highest level of estimation is based on an average
installed cost of an ozone system based on the nominal tonnage of the tower. An
installed cost of $10/kW ($36/ton) is typical for smaller systems. As the ozone
generators get larger, the cost per ton can drop. An average chemical treatment
program cost is $10/ton per year while an average ozone treatment will cost
around $2/ton per year. The cost of make-up water and disposal of blowdown can
vary widely and should be obtained for the particular cooling tower application
under consideration. In addition, local energy costs should be used for the
ozone energy consumption. The estimated costs and savings for the Utility Annex
cooling tower system are listed in Table 2.
Table 2. Estimated Cooling
Tower System Operating Information
|
|
Existing
system |
Ozone
system |
Difference
|
|
Operating cost |
$ 164,680/yr |
$ 40,215/yr |
$ 124,465/yr |
|
Ozone equipment cost |
not applicable |
$ 320,500 |
($320,500) |
|
Annual water use |
59,130,000 gal |
30,894,200 gal |
28,235,800 gal |
Life-Cycle Cost
The
estimates from the above calculations are to use a 690 gr/hr ozone generator.
Annual savings are estimated to be $124,465. Using the Building Life-Cycle Cost
software (BLCC 4.20-1995) available from the National Institute of Standards
and Technology (NIST), the total life-cycle cost for the ozone technology is $663,850
compared to a life-cycle cost of $1,463,555 for the conventional chemical
treatment program. A life cycle of 10 years was used in this analysis. The
comparison report from the BLCC software is illustrated in Figure 7. The
resulting net present value (NPV) is determined to be $799,705 and the
savings-to-investment ratio (SIR) is 3.5. More information on Federal
life-cycle costing and the BLCC software can be found in Appendix B.
 Fig.
7. Building Life-Cycle Cost (BLCC)
Fig.
7. Building Life-Cycle Cost (BLCC)
Implementation and Post-Implementation
Experience
The
ozone system installed at the Utility Annex has a generation capacity of 600
gr/hr. For comparative purposes, the actual costs and savings reported by
Tierney and Mott are identified in Table 3. The overall savings was determined
to be $100,012/year. Experience at the Utility Annex cooling towers has shown
that ozone treatment is indeed a viable water treatment method for cooling
towers. The idea that zero blowdown can be practiced is not feasible, since the
calcium levels will eventually get too high and scale will form. At 60 to 80
cycles, the cooling towers were 60% plugged with scale in 8 months. In
addition, the ozone injection circuit was plagued by the same problem and was
difficult to keep on line. This forced the operators to reduce the
concentration cycles between 10 and 20. Research indicated that they could
increase the concentration cycles between 30 and 40, which is where they are
now.
Table 3. Reported(a)
Cooling Tower Operating Information
|
|
Existing
system |
Ozone
system |
|
Operating cost
|
$ 161,484/yr |
$ 61,472/yr |
|
Ozone equipment cost |
not applicable |
$ 330,000 |
|
Annual water use |
53,290,000 gal |
35,690,000 gal |
|
(a) Reported from telephone interview with
site personnel. |
||
The
ozone generator failed several times due to excessive heat but was covered by
the manufacturer's warranty. To remedy the failure conditions of the ozone
unit, an air-conditioned enclosure was built to remove some of the cooling load
on the ozone generator's cooling system. This points out the need to have the
cooling system for the ozone generator serviced regularly to reduce failures in
the unit and to consider the cost of enclosing and cooling the unit if it must
operate in a high temperature environment.
Ozone
injection systems are susceptible to scale build-up due to the dry ozone/air
stream coming into contact with the mineral-saturated cooling tower water. This
problem was solved by injecting potable water (which is not mineral-saturated)
at the site of ozone injection.
Overall,
the results are good. The reduction in blowdown, make-up water, and chemical
costs usually will provide a simple payback time of less than six years.
This
case study concerns a system of two cooling towers with a capacity of 300 tons
each, located at the Lockheed Martin Electronics and Missiles Ocala Operation
in Ocala, Florida. Data were taken from a paper written and presented at the
DOE Pollution Prevention Conference XI in Knoxville, Tennessee, on May 16, 1995
(See "Who Is Using the Technology" for a contact at Lockheed Martin).
The
Lockheed Martin Electronics and Missiles Ocala Operation is responsible for the
production of electronic assemblies, printed circuit boards, and wiring
harnesses for space exploration, defense weapon systems, and defense
communication systems. The cooling towers support a variety of test and
production equipment and also support secondary cooling of HVAC systems.
The
cooling tower system consists of two conventional Marley counterflow cooling
towers with an operating capacity of 500 gallons each. The towers operate with
an influent water temperature of 85 F (29.4 C) and an effluent temperature of
approximately 75 F (23.8 C), for an overall temperature drop of 10 F (5.6 C).
The facility was not connected to a public works wastewater treatment facility,
so the blowdown water had to be transported offsite for disposal, at an annual
cost of $45,360.
The
cooling towers had an annual make-up water volume of 2.482 million gallons.
Since the installation was not connected to an outside water source, the source
of make-up water was treated wastewater recycled from the manufacturing
process. This make-up water had a total organic carbon (TOC) content that was
greater than 1500 ppm. This high TOC concentration resulted in a large chemical
demand in treating the cooling tower water, which was reflected in the overall
chemical treatment costs. The water was soft (~=50 ppm as CaCO3) and
contained ferrous sulfate from the manufacturing process. Poor system control
resulted in either excessive chemical use or insufficient chemical feed, with
subsequent scale formation requiring acid cleaning. The tower required acid
cleans several times a year and the chiller condensers were cleaned at least
twice during the summer months due to biofilm growth that resulted in excessive
pressure head.
The
existing multi-chemical treatment program consisted of the application of
chlorine gas, additional biocides, and corrosion inhibitors. The total annual
chemical costs were $24,733.
The
savings data identified in Table 4 were generated by personnel in charge of
system operation. Significant savings were achieved in all elements of the
process: labor, energy, chemical, and blowdown disposal.
Table 4. Operating Cost
Comparison for Cooling Water System Per Year
|
Item
|
Chemical
Treatment |
Ozone
Treatment |
|
Electrical operation |
$0 |
$2,592 |
|
Chemicals |
$18,613 |
$0 |
|
Labor |
$9,360 |
$2,808 |
|
Blowdown hauling |
$45,360 |
$4,536 |
|
Chlorine gas |
$6,120 |
$0 |
|
Power consumption |
$118,715 |
$47,479 |
|
Total cost/year |
$198,168 |
$57,415 |
Savings
with ozone treatment were $140,753/year with an NPV of $1,072,235 and an SIR of
31.9.
In
this situation, prior to the installation of the ozone system, the costs and
maintenance were high enough to cause the facility to examine alternative
methods for cooling tower water treatment. The result was a decision to use
ozone for the treatment of the water. A proposal from REZ-TEK International,
Inc. was obtained in 1993 for the installation. In February 1994, a REZ-TEK
model S-1230 was installed and put into service. The model S-1230 produces 0-30
grams of ozone per hour and sold for around $35,000. The ozone system came
completely self-contained with a foot print of 37 inches by 30 inches and a
height of 55 inches. The appropriate electric service was already in place, so
the installation of the unit took one day. It should be noted that the time and
cost of installation will increase if the appropriate electrical service is not
available.
During
initial start-up of the system, a significant amount of suspended particles
were observed. This was from the precipitation of the minerals in the water and
was an expected phenomenon. In this application, the suspended solids were
removed by application of hydrogen peroxide as a make-up water pretreatment.
Addition of ferrous sulfate was also eliminated from the make-up water, and the
sump water was filtered.
The
bacterial count was reduced three orders-of-magnitude, from one million to one
thousand colony-forming units (CFUs), and blowdown waste was reduced 90%. The
operator reported that no chemicals had been added to the cooling tower one
year after the ozone system was installed.
Labor
savings were reported qualitatively: "Maintenance operator was enabled to
alternate one chiller and remove waste heat from air conditioning and test
chambers. System has allowed the maintenance operator time to focus on the
other facility issues." An important aspect of this type of savings is
that it will free up maintenance staff to address other operation and
maintenance issues at the facility.
Corrosion
tests indicated that copper in the tower neither corroded nor pitted, while
iron showed 2.0 mils per year of corrosion and 0.37 mils per year of pitting.
It was reported that the corrosion effect of ozone was 50% of that of chlorine
treatment.
The
findings of the case study were very positive one year after installation and
start-up.
Much
excitement has been generated around this technology. Manufacturers and vendors
see a huge market; cooling tower operators see the potential costs savings,
environmental benefits, and reductions in maintenance and health hazards. As a
result, many players have appeared in the field along with a variety of
products, services, and performance claims.
With
each installation, more is learned about actual performance, cost, and
benefits. There have been reports of success and of failure. Manufacturers
indicate that many of the failures were due to poor design or inferior quality
ozone-generating equipment. Sometimes the application of ozone was
inappropriate due to the make-up water condition or the tower operating conditions.
In these situations, a traditional chemical treatment program will be more
effective.
There
are many reasons to consider ozone: when chemical costs are high or chemical
management is burdensome, when chemical water treatment is not effective, when
water and sewer charges are high or increasing, or when local regulations
require blowdown to be treated before discharge to surface waters.
Potential
users should carefully review their current and historic costs related to
cooling tower water treatment and the performance of their associated cooling
equipment. The guidance provided in this Technology Alert should help
indicate whether it would be worthwhile to consider the technology.
The
firms listed below were identified as manufacturers and suppliers of the
technology at the time of this report's publication. This listing does not
purport to be complete, to indicate the right to practice the technology, or to
reflect future market conditions.
American Ozone Systems, Inc. 1301 North Elston Avenue
Chicago, IL 60622 (312) 278-3000
Biozone Technologies 7 Old Dock Road
Yaphank, NY 11980 (516) 734-2696 or c/o MW Equipment, Inc.
(212) 643-7700 (attn: Dick Dabberdt)
Capital Controls Company , Inc. 3000 Advance
Lane
PO Box 211 Colmar, PA 18915 (215) 997-4030
Carus Chemical Company Ozone Systems
315 Fifth Street Peru, IL
61354 (815) 223-1500
Clear Water Technology, Inc. 850#E Capitolio
Way
San Luis Obispo, CA 93401 (805) 549-9724
Diversey Water Technologies, Inc. 7145 Pine
Street
Chagrin Falls, OH 44022 (800) 669-0053
EDC 3110 W. Story Rd. Irving, TX 75038
(214) 257-0322 (attn: Patrick Hunt)
Emery-Trailgaz Ozone Company 11501 Goldcoast
Dr. Cincinatti,
OH 45259-1643 (513) 530-7702
Hankin Atlas Ozone Systems, Ltd. 690 Progress
Avenue, Unit #12
Scarborough, Ontario M1H 3A6 Canada (416) 439-7860
Griffin Division of Ozonia North America
PO Box 330, 178 Route 46 Lodi, NJ 07644
(201) 778-2131
Marley Cooling Tower 7401 W. 129th
St. Overland Park, KS 66213
(913) 664-7614 (attn: Terri Robee)
Mitsubishi International Corporation 875
North Michigan Avenue,
Suite 3900, John Hancock Center Chicago, IL 60611
(312) 640-5647
Osmonics, Inc. 5951 Clearwater Drive
Minnetonka,
MN 55343-8990 (612) 933-2277
Ozonair International Corporation
903 Grandview Drive South San Francisco, CA 94080
(415) 952-9904
Ozone Research & Equipment Corp. 4953
West Missouri Ave. Phoenix, AZ 85301
(602) 931-7332
Ozone Technology Inc. 2113 Anthony Dr. Tyler,
TX 75701
(903) 581-2060
Ozonia North America 2924 Emerywood Parkway
PO Box 70145
Richmond, VA 23229
(804) 756-0500
Ozotech, Inc. 2401 Oberlin Rd. Yreka, CA
96097
(916) 842-4189
Ozone Technology Incorporated 2113 Anthony
Dr. Tyler, TX 75701
(903) 581-2060
Panlmatic Company 79 Bond Street Elk Grove
Village, IL 60007
(708) 439-4454
Sumitomo Precision Products Co., Ltd. 345
Park Ave. New York, NY 10154
(212) 826-3634
PCI Ozone & Control Systems, Inc. One
Fairfield Crescent West Caldwell, NJ 07006
(201) 575-7052
REZ-TEK International Corp. 15 Avenue E
Hopkinton, MA 01748
(800) 770-8554 (attn: Jim Daly)
Wheelabrator Engineered Systems, Inc. P.O.
Box 36, 441 Main Street Sturbridge, MA 01566
(508) 347-7344
Zelsman and Associates 329 Nebraska Ave.
Longwood, FL 32750
(407) 831-6268 (attn: Jack Zelsman)
The
list below is a partial list of Federal-sector contacts, agencies, and
locations that already have the new technology installed and operating. Many of
the listed Federal energy managers are knowledgeable about ozone for cooling
tower water treatment. The reader is invited to ask questions and learn more
about the new technology.
Kennedy Space Center (EG&G)
Kennedy Space Center, FL
Dan Tierney (407) 867-1190
Lewisburg Penitentiary
Lewisburg PA
Lou Brememen (717) 523-1251 x418
Lockheed-Martin
Ocala, FL
Arvind Patel (904) 687-5683
Martin-Marietta
Oak Ridge, TN
Terry Copeland (615) 574-1550
McDonnell-Douglas Space System
Kennedy Space Center, FL
Jose Rodriguez (407) 867-5141
NASA Houston
Houston, TX
Mark Watts (713) 666-2828
United States Post Office
Manchester, NH
Ron Bruzenski (603) 644-4071
The
documents listed below were used in the preparation of this Technology Alert
and may be of further use to anyone considering application of cooling tower
ozone treatment. A list of pertinent associations and organizations is also
provided.
User and third party field and lab test
reports and other technical publications:
1994
ASHRAE Handbook, Equipment Volume, Chapter 20, Cooling Towers, American
Society of Heating, Refrigerating, and Air Conditioning Engineers, Inc.
Aqua-Chem,
Inc. nd. "Ozone and the Environment." Aqua-Chem, Inc., Raleigh, North
Carolina.
Burda,
Paul A., Brian A. Healey, and Guna Selvaduray. 1993. "Performance and
Mechanisms of Cooling Tower Treatment by Ozone." Paper No. 488, presented
at Corrosion 93, the NACE Annual Conference and Corrosion Show. Pacific Gas and
Electric Company Technology Center, San Ramon, California.
Coppenger,
G. D., B. R. Crocker, D.E. Wheeler, 1989, Ozone Treatment of Cooling Water:
Results of a Full-Scale Performance Evaluation, Oak Ridge Y-12 Plant,
Martin Marietta Energy Systems, Inc.
Donohue,
J.M. 1972, Cooling Tower Treatment -- Where Do We Stand?, National
Association of Corrosion Engineers.
Dore,
M. 1985. "The Different Mechanisms of the Action of Ozone on Aqueous
Organic Micropollutants." In Proceedings of the International Ozone
Association Conference, London, November 13-14, 1985.
Echols,
Joseph T., and Sherman T. Mayne. 1990. "Cooling Tower Management Using
Ozone Instead of Multichemicals. ASHRAE Journal, June 1990.
Edwards,
H., P.E. Banks. 1987. "Ozone--An Alternate Method of Treating Cooling
Tower Water." Paper No. TP87-17, presented at the 1987 Cooling Tower
Institute Annual Meeting, New Orleans, February 25-27, 1987.
Electric
Power Research Institute (EPRI). 1992. Tech Application: Ozonation of
Cooling Tower Water. No. 3, EPRI Industrial Program - Environment and
Energy Management, Palo Alto, California.
HACH
Company. 1992. Water Analysis Handbook. 2nd Edition. HACH Company,
Loveland, Colorado.
Henley,
Mike. 1994. "Ozone Review: Ozone Finding Small Niche as Cooling Tower
Treatment." In Industrial Water Treatment, March-April 1994.
Kaur,
K., T.R. Bott, and B.S.C. Leadbeater. 1992. "Effect of Ozone on Pseudomonas
Fluorescens." In Biofilms--Science and Technology, L.F. Malo et
al. eds., pp. 589-94. Kluwer Academic Publishers, Netherlands.
Kenney,
Ray. 1983, Ozonation as Cooling Tower Water Treatment: A Pilot Study,
IBM Technical Report TR 20.0430
Legube,
B., J-P. Croue, D.A. Reckhow, M. Dore. 1985. Ozonation of Organic Precursors
Effects of Bicarbonate and Bromide, In Proceedings of the International
Ozone Association Conference, London, November 13-14, 1985
Masschelein,
W.J. 1985. Mass Transfer of Ozone Through Bubbling and Chemical Reactions in
Water, In Proceedings of the International Ozone Association Conference,
London, November 13-14, 1985.
Miltner,
R. J., H. M. Shukairy, R. S. Summers, Disinfection By-Product Formation and
Control by Ozonation and Biotreatment, Journal of American Water Works
Association, V84 n11 pp. 59-62, November 1992.
Montgomery,
James M., Consulting Engineers, Inc. 1985. Water Treatment Principles and
Design. John Wiley & Sons, New York.
Nebel,
Carl. 1985, "The Oxidation Mechanism of the Oxyozonsynthesis
Process," In Proceedings of the International Ozone Association
Conference, London, November 13-14, 1985.
Nebel,
Carl. 1994. "Design Consideration for Ozone Water Treatment Systems in
Cooling Towers." Paper No. TP94-07, presented at the 1994 Cooling Tower
Institute Annual Meeting, Houston, Texas, February 13-16, 1994. PCI Ozone &
Control Systems, Inc.
Nebel,
Carl. 1995, Design of Ozone Systems for Cooling Towers, Engineered
Systems, April 1995.
Ozone,
Kirk-Othmer Encyclopedia of Chemical Technology, Volume 16, Third Edition,
Copyright 1981, John Wiley and Sons, Inc.
Pacific
Gas & Electric (PG&E). 1991. Evaluation of Ozone Technology for
Chemical Treatment Replacement in Cooling Towers (Power Plant Systems): Final
Report. Report 006.2-90.6, Pacific Gas and Electric Company, San Ramon,
California.
Patel,
Arvind B. 1995. Pollution Prevention in Cooling Tower Water Treatment. DOE
Pollution Prevention Conference XI, Knoxville, Tennessee, May 16, 1995.
Pope,
Daniel H., Lawrence W. Eichler, Thomas F. Coates, Jeffrey F. Kramer, and
Reginald J. Soracco. 1984. "The Effect of Ozone on Legionella
pneumophila and Other Bacterial Populations in Cooling Towers." Current
Microbiology 10:89-94.
Pryor,
A.E., T.E. Buffum, "A New Practical Index for Predicting Safe Maximum
Operating Cycles in Ozonated Cooling Towers," Ozone Science &
Engineering, 17, 71-96, 1995.
Puckorius,
Paul R. 1993. "Ozone Use in Cooling Tower Systems - Current Guidelines -
Where It Works." Ozone Science & Engineering 15:81-93.
Stumm,
W., J.J. Morgan, "Aquatic Chemistry." 2nd Ed., John Wiley & Sons,
New York, NY, 1981.
Soeyink,
V.L., D. Jenkins, "Water Chemistry," pp76-79, J. Wiley & Sons,
Inc., New York, NY, 1980.
Tierney,
D.J. Cooling Tower Ozone Treatment at Kennedy Space Center.
EGG-8600/BOC-125 Tierney 407-867-1190.
Tierney,
D.J., R.A. Mott. Ozone V. Chemical Treatment of Cooling Towers at Kennedy
Space Center: A Progress Report. Tierney 407-867-1190.
Tierney,
D.J., E.S. Feeney, R.A. Mott. Case History: Performance Evaluation of Ozone
Cooling Water Treatment at Kennedy Space Center. Tierney 407-867-1190.
Wattinger,
Ralph. 1993. "Ozone: An Environmentally Beneficial Means of Treating
Cooling Tower Water." Presented at the 2nd International Energy and
Environmental Congress, Minneapolis, Minnesota, August 4-5, 1993. REZ-TEK
International, Inc., Mountaindale, New York.
Weisstuch,
A., D.A. Carter, C.C. Nathan. 1971, Chelation Compounds as Cooling Water
Corrosion Inhibitors, National Association of Corrosion Engineers.
Utility, Information Service, or Government
Agency Technology Transfer Literature:
City
of San Jose. 1992. Water Conservation Guide for Cooling Towers.
Environmental Services Department, City of San Jose, California.
Electric
Power Research Institute (EPRI). 1992. TechApplication: Ozonation of Cooling
Tower Water. No.3, EPRI Industrial Program--Environmental and Energy
Management.
Electric
Power Research Institute (EPRI). 1992. Ozonation of Cooling Tower Water: An
Alternative Treatment Technology. BR-100426, Electric Power Research
Institute, Palo Alto, California.
International
Ozone Association. 1994. Ozone News 22:5 (1994).
Associations and Professional Organizations:
American Society of Heating, Refrigerating
and Air-Conditioning Engineers, Inc. (ASHRAE)
1791 Tullie Circle, N.E. Atlanta, GA 30329
Cooling Tower Institute P.O. Box 73383
Houston, Texas 77273
Phone: (713) 583-4087 Fax: (713) 537-1721
Electric Power Research Institute (EPRI) 3412
Hillview Avenue P.O. Box 10412 Palo Alto, CA 94303
Phone: (415) 855-2411
International Ozone Association
Pan American Group 31 Strawberry Hill Avenue Stamford, CT 06902
Phone: (203)348-3542 Fax: (203)967-4845
National Association of Corrosion Engineers,
International Products Division
P.O. Box 218340 Houston, Texas 77218
Phone: (713) 492-0535 Fax: (713) 492-8254
General Contacts
Ted Collins
New Technology Demonstration Program
Program Manager
Federal Energy Management Program
U.S. Department of Energy
1000 Independence Avenue, SW, EE-92
Washington, DC 20585
(202) 586-8017
Fax: (202) 586-3000
theodore.collins@hq.doe.gov
Steven A. Parker
Pacific Northwest National
Laboratory
P.O. Box 999, MSIN: K5-08
Richland, Washington 99352
(509) 375-6366
Fax: (509) 375-3614
steven.parker@pnl.gov
Technical
Contact
Steven
A. Parker
Pacific Northwest National Laboratory
P.O. Box 999, MSIN: K5-08
Richland, Washington 99352
(509) 375-6366
Fax: (509) 375-3614
steven.parker@pnl.gov

Produced for the U.S. Department of Energy by the Pacific Northwest National
Laboratory
December 1995